
- #Oxidation number of all elements in periodic table how to
- #Oxidation number of all elements in periodic table free
Superoxides and Peroxides are compound of oxygen in which atoms of oxygen are linked directly to one another. However, there are exceptions in case of peroxides, superoxides, and oxygen bonded with fluorine. Oxygen in most of its compounds state has an oxidation number of -2.
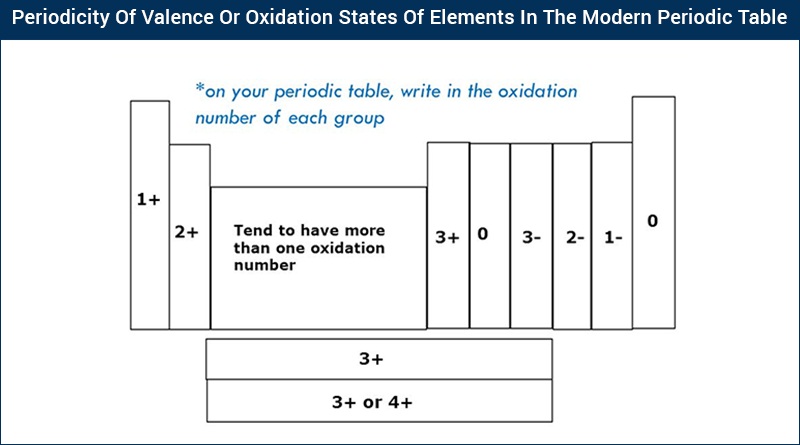
However, aluminium in all its compound form has oxidation number +3. Similarly, all alkaline earth metals have an oxidation number of +2. All alkali metals in the compound form will have oxidation number +1. Similarly, Mg 2+, Fe 3+ ion, Cl – ion, O 2– ion will have charge +2, +3, –1, –2, respectively. For instance, Na + ion has the oxidation number +1. Ions having one atom bear oxidation number equal to the charge present on the ion.
#Oxidation number of all elements in periodic table free
Therefore, each atom in H 2, O 2, Na, Cl 2, O 3, P 4, S 8, Mg, etc in their free form has oxidation number zero. When the elements are present in free or its elemental state then the oxidation number of the particular element will be zero. Rules for Determination of Oxidation Number Rule 1 We will further understand this later with an example. Moreover, if in a molecule/compound/ion two or more elements are present then the average of all the atoms of the particular element is taken to find the oxidation number of the given element. Thus, it led to the formulation of a certain set of rules for the determination of an element in a particular compound or ion. However, it is difficult to make out which is more electronegative element than the other in any given compound or ion. The rules were set on the basis of the electron pair of a covalent compound and the electronegativity of an element. Oxidation number refers to the oxidation state of a particular element in a compound by following a set of rules. Later on, it will become clear that electron transfer is nothing but the simple descriptions of redox reactions. It is important to understand that assumptions on electron transfer are made just for keeping track or recordkeeping purpose only.
#Oxidation number of all elements in periodic table how to
Refer to the example below to see how to show charge on each atom as part of the reaction in atomic number. In the oxidation number method, we consider that a complete transfer of an electron from less electronegative to more electronegative atom takes place. The more practical method is the use of oxidation number to keep track of the electron shifts happening in the chemical reactions. It is important to keep track of the electron shifts in the reactions that involve the covalent compound formations. Refer to the example to understand this class of reactions: This reason is also applicable to other reaction examples as well. Therefore, it can be described as electron shift rather than electron transfer or electron loss where complete loss and gain of electrons occurs in Hydrogen and oxygen, respectively. However, when we study in depth about charge transfer we will understand that the reaction is only partial.


Redox Reactions as the Basis of Titrations.Redox Reactions and Electrode Potential.As a result of H 2 and O 2 are oxidized and reduced, respectively. Thus, we can clearly see that there is an electron transfer taking place in hydrogen and oxygen in the reaction. Similarly, the oxygen atom in O 2 is present in its zero state converts to di-negative state in H 2O. All of us know the reaction,Įven though it is not a very common reaction but we can notice that the H atom in the neutral state (zero) in H 2 converts to positive state after the formation of H 2O. Let us go through the article to understand oxidation numbers in details! One of the less known examples of electron transfer is when hydrogen and oxygen combine to form water. Therefore, we can determine oxidation numbers for atoms of any element irrespective of covalent or ionic bonding. However, oxidation numbers do not necessarily mean real charges on molecules. Oxidation number, also known as oxidation state, is used for determining how many electrons an atom has.


 0 kommentar(er)
0 kommentar(er)
